Blog
Blog
GxP Compliance Across Global Markets: Key Challenges and Best Practices
In the pharmaceutical and life sciences industry, GxP compliance serves as the foundation for ensuring product quality, patient safety, and […]
How to Create a Robust CSV Master Plan: A Step-by-Step Guide
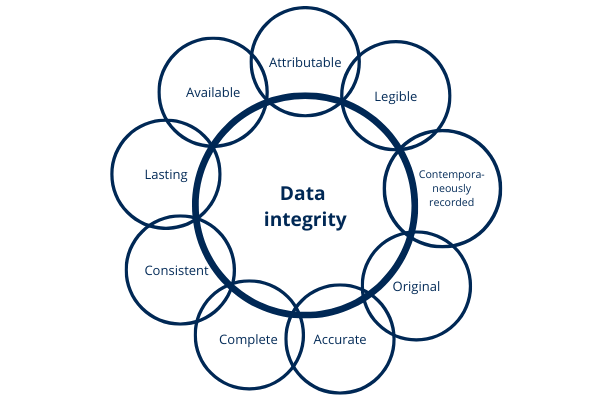
In the pharmaceutical and life sciences industry, compliance is not optional—it is mandatory. One of the critical pillars of compliance […]
The Role of Computerized System Validation (CSV) in Pharmaceutical Compliance
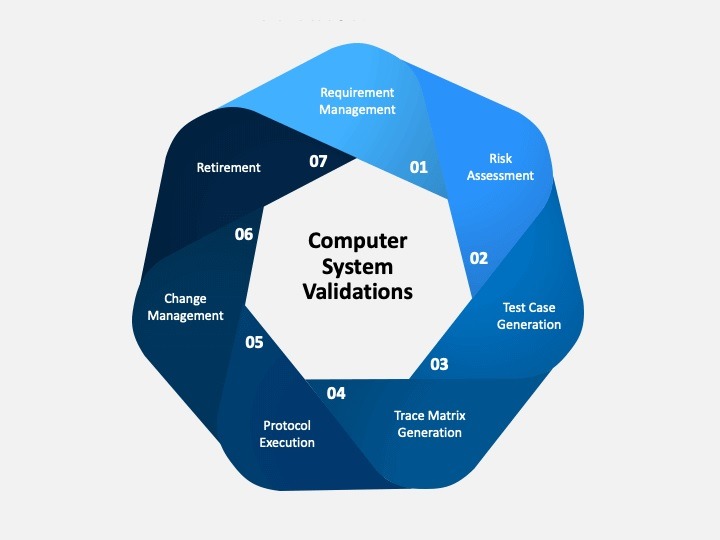
In the highly regulated pharmaceutical industry, ensuring the safety, efficacy, and quality of products is non-negotiable. With the growing integration […]